Frequently Asked Questions about COVID-19 Vaccines
Updated on February 4, 2022
As the COVID-19 vaccines are introduced, many people have questions about their development, safety, access, cost, and other common concerns. This page provides information about what scientists do and do not know yet about the vaccines, drawing from the World Health Organization (WHO), the Ministry of Health and Family Welfare, Government of India (MoHFW), the United States Centers for Disease Control and Prevention (CDC), and other trusted sources. Some of this language is used word for word, and other language is paraphrased. We would like to acknowledge the hard work of these organizations in compiling this information.
Getting vaccinated is one of many steps you can take to protect yourself and others from COVID-19. Other recommendations including the use of face masks, distancing and washing hands should continue to be followed to reduce your chance of being exposed to the virus or spreading it to others. Here is a illustrative guide to COVID-19 appropriate behaviours published by the MoHFW.
If you cannot find the information you are looking for on this page, you are invited to submit your own questions through this form and our team will look into the answers.
Getting a vaccine- eligibility, vaccine timing, and doses
Who is eligible for the COVID-19 vaccine?
UPDATED As of 3rd January 2022, children in the age-group 15-18 years and all adults over 18 years (including pregnant and lactating women) are eligible for the COVID-19 vaccine. However, only “Covaxin” is the approved vaccine for children in the age-group 15-18 years. Please check your local state guidelines for more information.
If you cannot pre-register online, please contact your local government health workers, who will refer you to the government COVID-19 Vaccination Center for on-the-spot registration, appointment, verification, and vaccination on the same day.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html, https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf and https://www.mohfw.gov.in/pdf/GuidelinesforCOVID19VaccinationofChildrenbetween15to18yearsandPrecautionDosetoHCWsFLWs&60populationwithcomorbidities.pdf
Who is eligible for the COVID-19 vaccine precaution dose?
NEW As of 10th January 2022, Health Care Workers and Front-Line Workers who have received two doses of the COVID-19 vaccine are eligible to receive another dose of the vaccine called the precaution dose. All persons aged 60 years and above with comorbidities and who have received two doses of the COVID-19 vaccine are eligible to receive the precaution dose based on their doctor’s advice. The order in which these precaution doses would be given are based on the completion of 9 months or 39 weeks from the second dose.
For more information, please visit: https://www.mohfw.gov.in/pdf/GuidelinesforCOVID19VaccinationofChildrenbetween15to18yearsandPrecautionDosetoHCWsFLWs&60populationwithcomorbidities.pdf
If I have already had COVID-19 and recovered, do I still need to get the COVID-19 vaccine?
Yes, you should get a COVID-19 vaccine, even if you’ve had COVID-19. The vaccine will still help in developing a strong immune response against the disease. But wait for 3 months after being infected with COVID-19 before getting the vaccine.
We do not know yet if people who have had COVID-19 will have enough of an immune response to protect them from getting COVID-19 again. Even if there is some protection, it is not known how long it will last. This is why people who have had COVID-19 should still get a COVID-19 vaccine.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
If I currently have COVID-19, should I get the vaccine now?
No, people with confirmed or suspected COVID-19 should be in isolation for at least 10 days. Having infected people at the vaccination site would increase the risk of spreading the virus. Since people with COVID-19 develop some immunity against the disease, the Ministry of Health and Family Welfare, Government of India (MoHFW, GoI) recommends deferral of vaccination for 3 months after recovery.
What if I get COVID-19 in between 2 vaccine doses?
For most people who have 1 dose of the vaccine, the disease is likely to be mild or moderate, depending on how many days after vaccination the virus exposure happens. If exposure occurs within 1 to 3 weeks of getting the first dose (of Covaxin or Covishield), the vaccine is unlikely to have an effect and is not expected to change the course of the infection. However, if a person tests positive after 3 weeks from their first dose, they are highly likely to only get a mild case of COVID-19.
Once a person gets COVID-19, their body starts producing antibodies to it. Therefore, experts suggest waiting 3 months after recovering from COVID-19 before getting the second dose. Getting the second dose is important to ensure an effective immune response against COVID-19 in the future.
So, while experts learn more and as people get vaccinated, it is important for everyone (vaccinated and unvaccinated) to continue wearing masks, washing our hands, and keeping physical distance from others to help stop this pandemic.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
Why do I need to get two doses of the vaccine?
Recent studies have shown that certain time frames between vaccine doses allow for the best immune response. This timing is different for each vaccine. This has to do with the different ways each vaccine works with your body’s immune system.
Covishield: Real-world studies of the Covishield vaccine show that waiting a longer time between doses led to a better immune response. So the time between the two doses has been increased to 12-16 weeks.
Covaxin: Scientists recommend that the second dose of the Covaxin vaccine be taken 4-6 weeks after the first dose.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
What is the right timing between COVID-19 vaccine doses?
Recent studies have shown that certain time frames between vaccine doses allow for the best immune response. This timing is different for each vaccine. This has to do with the different ways each vaccine works with your body’s immune system.
Covishield: Real-world studies of the Covishield vaccine show that waiting a longer time between doses led to a better immune response. So the time between the two doses has been increased to 12-16 weeks.
Covaxin: Scientists recommend that the second dose of the Covaxin vaccine be taken 4-6 weeks after the first dose.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
Do I need to get the same vaccine for the first and second dose of my COVID-19 vaccine?
Yes. The vaccines currently available in India are not interchangeable. So, you need to get the same second dose of the vaccine as the first one. Your vaccine registration (through the CoWIN portal) is also going to help to ensure that you get the same vaccine for both doses.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
Getting a vaccine- during and after your appointment
How can I stay safe from COVID-19 at vaccination centers?
- Keep at least 6 feet (2 meters) distance from others at the center.
- Wear a 3-layered medical mask or N95 mask over your nose and mouth. If not possible, double mask (wear a cloth mask over a disposable surgical mask).
- Do not touch your face or any surfaces. Do not shake hands.
- Sanitize your hands often.
- Avoid talking to others.
- Plan ahead. Register in advance, if possible, and understand the timing of your next appointment.
- Stay at home and reschedule your appointment if you have symptoms for COVID-19, or, have COVID-19.
For more information, please visit: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
Do I need to wear a mask and avoid close contact with others if I have received two doses of the vaccine?
Yes. While experts learn more about the protection that COVID-19 vaccines provide under real-life conditions, it will be important for everyone to continue using all the tools available to us to help stop this pandemic. These tools include covering your mouth and nose with a mask, washing hands often, and staying at least 2 meters away from others. Together, the COVID-19 vaccine and everyone following the COVID-19 safety guidelines will offer the best protection from getting and spreading COVID-19. Experts need to understand more about the protection that COVID-19 vaccines provide before deciding to change recommendations on steps everyone should take to slow the spread of the virus that causes COVID-19.
COVID-19 vaccines are effective at preventing COVID-19 disease, especially severe illness and death. But we’re still learning how well COVID-19 vaccines keep people from spreading the disease. Moreover, we’re still learning how effective the vaccines are against variants of the virus that causes COVID-19. Early data show the vaccines may work against some variants but could be less effective against others.
A small number of people may still develop infection, but it is likely to be less severe. And hence it is necessary to wear a mask, wash hands and stay away from crowds. Until we know more about the effectiveness of the vaccine against the variants, and until a majority of the population is vaccinated to reach herd (or community) immunity, it is necessary to continue to follow the COVID-19 prevention protocols.
Source: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html
Are there side effects to receiving the vaccine?
COVID-19 vaccination will help protect you from getting COVID-19. You may have some side effects, which are normal signs that your body is building protection. Common side effects include pain and swelling at the site of injection, and flu-like symptoms such as fever, chills, tiredness and headache. These should go away in a few days.
It has been seen that some people can develop allergic reactions after getting the COVID-19 vaccine. If you have ever had a severe allergic reaction to other vaccines or injectables, it is recommended that you speak to your doctor before getting vaccinated.
Do fully vaccinated people in India need to continue wearing masks in social settings?
Yes. While experts learn more about the protection that COVID-19 vaccines provide under real-life conditions, it is important for everyone to continue using all the tools available to help stop this pandemic. These tools include covering your mouth and nose with a mask, washing hands often, and staying at least 6 feet or 2 meters away from others. Together, the COVID-19 vaccine and everyone following COVID-19 safety guidelines will offer the best protection from getting and spreading COVID-19.
COVID-19 vaccines are effective at preventing COVID-19 disease, especially severe illness and death. But we are still learning how well COVID-19 vaccines keep people from spreading the disease. We are also still learning how effective the vaccines are against variants of the COVID-19 virus (variants are versions of the virus with slight changes, or mutations, that sometimes affect how contagious or severe the virus can be). Early data show the vaccines may work against some variants but could be less effective against others.
Experts need to understand more about the protection that COVID-19 vaccines provide before deciding to change recommendations on what everyone should do to slow the spread of COVID-19. So, while experts learn more and as people get vaccinated, it is important for everyone to continue wearing masks, washing our hands, and keeping physical distance from others to help stop this pandemic.
Adapted from: https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html
Vaccine Safety
Can I get COVID-19 from the vaccine?
No, the different types of vaccines have different materials in them that will signal our bodies to produce the proteins or antibodies to protect us from the virus. NONE of these vaccines contain the active virus.
The immune response that is initiated in the body might lead to symptoms, such as fever. This does not mean the person is infected with the virus. Learn more on how Covishield (Serum Institute) and Covaxin (Bharat Biotech) vaccines work.
It typically takes a few weeks for the body to build immunity after vaccination. That means it’s possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and get sick. This is because the vaccine has not had enough time to provide protection.
How do we know the vaccine is safe?
The most commonly used vaccines we have today have been in use for decades, with millions of people receiving them safely every year. As with all these successful vaccines, COVID-19 vaccines have also undergone extensive and rigorous testing before being approved. Scientists around the world have been working since early 2020 to develop the current possible COVID-19 vaccines and go through all of the testing processes to ensure they are safe. The vaccine safety is also monitored after the vaccines have been introduced which is called post-market surveillance. This ensures that the vaccines will continue to meet the same quality, safety and performance requirements as when they were initially introduced in the market.
Is it safe for me to get the vaccine if I am pregnant or breastfeeding?
The vaccines being used under the national vaccination program are found to be safe and effective. Based on how these vaccines work in the body, experts believe they are unlikely to pose a risk for people who are pregnant. The National Technical Advisory Group on Immunization (NTAGI) has recommended that “pregnant women may take any one of the two Covid-19 vaccines and lactating women are also eligible for jabs at any time before and after delivery.” This recommendation is based on the emerging evidence which shows that benefits of COVID-19 vaccination during pregnancy far outweigh the risk associated with contracting COVID infection during pregnancy (like increased risk for severe illness, preterm birth). However, it’s important that pregnant women make an informed choice.
For more information, please visit: https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
Are risks of COVID-19 vaccination more than its benefits for a pregnant/lactating woman?
No, the very real benefits of vaccinating pregnant and lactating women seem to far outweigh any theoretical and remote risks of vaccination. Lactating women are also considered for Covid vaccine as there are no known adverse effects on the neonate who is breastfeeding. In fact, there is a possibility of passage of protective antibodies to the child, which may have a beneficial effect.
For more information, please visit: https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
Which vaccines should I take if I am pregnant?
National Technical Advisory Group on Immunization (NTAGI) recommends that pregnant women may take any one of the two Covid-19 vaccines (Covishield or Covaxin). You must consult your doctor about the choice of vaccine in particular case. Both COVISHIELD and COVAXIN can be used during pregnancy or lactation.
For more information, please visit: https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
Can women who are taking contraceptives and women planning to get pregnant get vaccinated?
Yes, women taking contraceptives can certainly get vaccinated. Pregnant women fall in the vulnerable group in terms of risk of serious disease in case of exposure. It might be safer to be fully immunized before conceiving.
For more information, please visit: https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
Does getting the vaccine affect my future fertility and the chances of getting pregnant?
No, there is no evidence or no indications so far that the COVID vaccines impact fertility.
For more information, please visit: https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
What should pregnant woman consider before getting the vaccine?
Expectant woman may consider discussing the following with their doctor/health care provider to guide them to make their decision:
- Likelihood of exposure to COVID-19, risks of COVID-19 to them and potential risks to her and fetus
- Benefits of getting vaccinated
- Information about the type of vaccine and known side effects of the vaccine.
For more information, please visit: https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
What are the reasons someone should not get the COVID-19 vaccine?
There are a few contraindications or reasons why someone should talk to a doctor before getting a COVID-19 vaccine:
- If people have a history of:
- Anaphylactic or allergic reaction to a previous dose of COVID-19 vaccine
- Immediate or delayed-onset anaphylaxis or allergic reaction to vaccines or injectable therapies, pharmaceutical products/medicines, food items, etc.
- Pregnancy:
- Pregnant women have not been part of any COVID-19 vaccine clinical trial so far. Therefore, women who are pregnant or not sure of their pregnancy status should not get a COVID-19 vaccine at this time. As more data emerges, this may change.
- Temporary reasons to delay getting the COVID-19 vaccine are if someone:
- Had COVID-19 recently (vaccination should be delayed for 3 months after recovering from COVID-19)
- Has active symptoms of COVID-19 infection (get tested for COVID-19)
- Has COVID-19 and has been given monoclonal antibodies or convalescent plasma
- Is acutely unwell and hospitalized (with or without intensive care) due to any illness
After their illness is over and after an appropriate amount of time, someone who had one of these conditions can get a COVID-19 vaccine.
If you are not certain if you or someone in your household should get the vaccine, talk to
a doctor.
For more information, please visit: https://www.mohfw.gov.in/COVID_vaccination/vaccination/faqs.html
Are the COVID-19 vaccines protective against newer strains / mutated virus of SARS-CoV2?
The body responds to vaccination by making more than one type of antibodies to virus parts including spike protein. Therefore, vaccines continue to reduce a person’s risk of contracting the virus that cause COVID-19, including the Delta variant. Vaccines are highly effective against severe illness, but the Delta variant causes more infections and spreads faster than earlier forms of the virus that causes COVID-19.
Top Things You Need to Know:
- Variants are expected. The best way to slow the emergence of new variants is to reduce the spread of infection by taking measures to protect yourself including getting a COVID-19 vaccine when available.
- Vaccines keep you from getting sick, being hospitalized, or dying from COVID-19.
- All COVID-19 tests can detect all variants, but they will not tell you which variant you have.
For more information: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant.html and https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
Why are people getting infected even after vaccination?
COVID-19 vaccines are effective. However, a small number of vaccinated people may still get COVID-19, but it is likely to be less severe. That is why it is still necessary to wear a mask, wash hands, and stay away from crowds.
Why a vaccinated person might get COVID-19:
- It’s possible a person could have been infected just before or just after vaccination. It typically takes about 2 weeks for the body to build protection after the second dose of vaccination. Thus, a person can get sick if the vaccine has not had enough time to provide protection.
- The virus that causes COVID-19 is evolving, and new variants of the virus are spreading. Current data suggest that COVID-19 vaccines offer protection against most variants. However, some variants might cause illness in some people after they are fully vaccinated.
- A small percentage of people who are fully vaccinated will still get COVID-19 if they are exposed to the virus that causes it. These are called “vaccine breakthrough cases.” This means that while people who have been vaccinated are much less likely to get sick, it may still happen. Experts continue to study how common these cases are.
The overall risk of hospitalization and death among fully vaccinated people will be much lower than among unvaccinated people with similar risk factors.
Should one avoid taking vaccine during and around menstruation?
UPDATED The time period around menstruation is no contraindication to taking vaccines and like other vaccines, COVID-19 vaccine can be taken at any time of the monthly period.
One study reported that women who received both doses of a COVID-19 vaccine in a single menstrual cycle found that their period arrived a few days late. However, their periods were back to normal after a couple of months, so the change was only temporary. Therefore, the study concluded that there is no population-level clinically meaningful change in menstrual cycle length associated with COVID-19 vaccination.
For more information, please visit: https://www.gavi.org/vaccineswork/how-covid-19-vaccines-affect-menstrual-cycle
and https://www.mohfw.gov.in/pdf/FAQsCOVID19vaccinesvaccinationprogramWebsiteupload.pdf
Does Covishield (Oxford/AstraZeneca) vaccine cause blood clots?
Covishield is NOT associated with an increased overall risk of blood clotting disorders. There have been very rare cases of unusual blood clots accompanied by low levels of blood platelets. They symptoms associated with the rare blood clots that require prompt medical treatment include: breathlessness; pain in the chest or stomach; swelling or coldness in a leg; severe or persistent headaches or blurred vision; or tiny blood spots under the skin beyond the site of the injection. One should seek urgent medical attention if they have any of these symptoms in the weeks after their injection.
Serious side effects from vaccines are rare but they do occur, with any vaccine and for any disease. However, we are witnessing the largest mass vaccination campaign in history. Given the high number of people being vaccinated and the attention focused on the vaccines and the pandemic, some rare reactions are to be expected. It remains to be seen if similar concerns will be raised over other COVID-19 vaccines, but given that AstraZeneca’s was the first to be approved and has been given to far more people than any others, any rare adverse events are more likely to show up simply because of the sheer volume of people to have received it.
Source: https://www.gavi.org/vaccineswork/what-blood-clotting-disorder-astrazeneca-vaccine-has-been-linked
Vaccine Basics-vaccine development and how they work
What are the ingredients in vaccines?
Today’s vaccines use only ingredients that are safe and effective. Each ingredient in a vaccine serves a specific purpose. For example, vaccine ingredients may:
- Help provide immunity (protection) against a specific disease
- Help keep the vaccine safe and long lasting
- Be used during the production of the vaccine
Ingredients provide immunity-
Vaccines include ingredients to help your immune system respond and build immunity to a specific disease. For example:
- Antigens are very small amounts of weak or dead germs that can cause diseases. They help your immune system learn how to fight off infections faster and more effectively. The flu virus is an example of an antigen.
- Adjuvants, which are in some vaccines, are substances that help your immune system respond more strongly to a vaccine. This increases your immunity against the disease. Aluminum is an example of an adjuvant.
- Messenger ribonucleic acid (mRNA), in some new COVID-19 vaccines, is the active component that generates an immune response in the recipient.
Ingredients keep vaccines safe and long lasting-
Some ingredients help make sure a vaccine continues to work like it’s supposed to and that it stays free of outside germs and bacteria. For example:
- Preservatives protect the vaccine from outside bacteria or fungus. Today, preservatives are usually only used in vials (containers) of vaccines that have more than 1 dose. That’s because every time an individual dose is taken from the vial, it’s possible for harmful germs to get inside. Most vaccines are also available in single-dose vials and do not have preservatives in them.
- Stabilizers, like sugar or gelatin, help the active ingredients in vaccines continue to work while the vaccine is made, stored, and moved. Stabilizers keep the active ingredients in vaccines from changing because of something like a shift in temperature where the vaccine is being stored.
Ingredients used during the production of vaccines-
Some ingredients that are needed to produce the vaccine are no longer needed for the vaccine to work in a person. These ingredients are taken out after production so only tiny amounts are left in the final product. The very small amounts of these ingredients that remain in the final product aren’t harmful. Examples of ingredients used in some vaccines include:
- Cell culture (growth) material, to help grow the vaccine antigens.
- Inactivating (germ-killing) ingredients, like formaldehyde, to weaken or kill viruses, bacteria, or toxins in the vaccine.
- Antibiotics, like neomycin, to help keep outside germs and bacteria from growing in the vaccine.
The claim that these vaccines contain a microchip or tracker is FALSE. The claim that these vaccines contain mercury is also FALSE.
Composition of Covishield includes inactivated adenovirus with segments of Coronavirus, Aluminium Hydroxide Gel, L-Histidine, L-Histidine Hydrochloride Monohydrate, Magnesium Chloride Hexahydrate, Polysorbate 80, Ethanol, Sucrose, Sodium Chloride, and Disodium Edetate Dihydrate (EDTA).
Composition of Covaxin includes inactivated Coronavirus, Aluminum Hydroxide Gel, TLR 7/8 Agonist, 2-Phenoxyethanol and Phosphate Buffered Saline [NKA1].
For more information about the ingredients and possible allergens, please ask your provider before getting the vaccine.
Why was the COVID-19 vaccine developed so much faster than other vaccines?
The vaccine process is happening faster because vaccine research and development, clinical trials, manufacturing, and plans for distribution are taking place at the same time. This method removes delays that occur when these processes are carried out one after the other. Steps to ensure safety have not been eliminated.
How are vaccines tested?
Vaccines go through an intensive testing process that includes careful examination of the vaccine and its ingredients. These tests evaluate the safety of the vaccine and how well it prevents a disease. Tests are first done in research labs, and then if the vaccine proves to be effective and safe, researchers can apply to start clinical trials.
Clinical trials typically involve several thousand healthy volunteers in three phases, with increasing numbers of participants in each phase. Trials in all phases have to follow strict safety regulations that are set by national regulatory authorities that prioritize participant safety. When vaccine manufacturers apply for approval for their vaccine, the results of all clinical trials are considered.
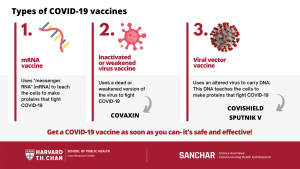
How do the different COVID-19 vaccines work?
Multiple COVID-19 vaccines are being developed, tested, and approved. They are all meant to teach the body’s immune system to safely recognize and block the coronavirus. The vaccine types include:
- Inactivated or weakened virus vaccines, which use a form of the virus that has been inactivated or weakened so it doesn’t cause disease, but still creates an immune response. For example: COVAXIN
- Viral vector vaccines, which use an altered virus to carry the genetic code (such as DNA) and create a protein that prompts an immune response, without causing COVID-19. For example: COVISHIELD
- mRNA vaccines, which contain “messenger RNA” to make a coronavirus spike protein. This protein alone cannot cause COVID-19.Our cells use this mRNA to make the viral protein that causes our immune systems to make antibodies that fight COVID-19. For example: PFIZER
Please follow the links below for more information:
Ministry of Health & Family Welfare. Frequently Asked Questions on COVID-19 vaccine.
Center for Disease Control and Prevention
New York Times COVID-19 Vaccine Resources
Ministry of Health & Family Welfare. COVID-19 operational guidelines. Published 28 December 2020.
This material was curated by Viswanath Lab of Harvard Chan School of Public Health and the Dana-Farber Cancer Institute (DFCI) with the help of the Health Communication Core of Dana-Farber/Harvard Cancer Center (DF/HCC). These are not the official views of Harvard Chan or DFCI. For any questions, comments or suggestions reach out to rpinnamaneni@hsph.harvard.edu.